Here’s an important water math practice problem. First, try to solve it without looking at the answer. If you don’t know how to solve it, read the following step-by-step solutions carefully until you understand it. Moreover, you may want to enroll in this online water math course, if you need help.
Above all, take your time, and don’t rush through it. Certainly, the most important thing is to learn how to solve this type of math problem before you take the operator certification test.
PRACTICE QUESTION
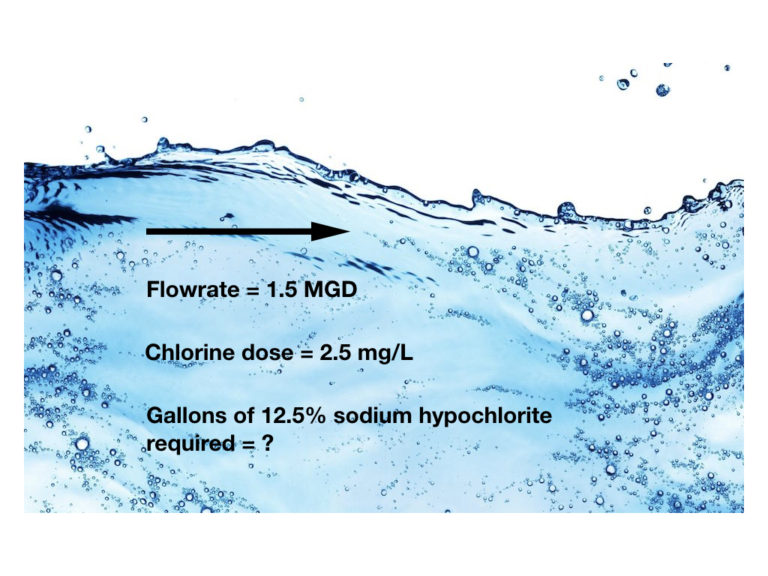
How many gallons of 12.5% sodium hypochlorite solution are required each day to disinfect a water supply flowing at a rate of 1.5 MGD, if the desired chlorine dose is 2.5 mg/L? The specific weight of 12.5% sodium hypochlorite solution is 10.2 lbs/gallon.
ANSWER
Step 1: LIST ALL THE DATA GIVEN
KNOWN
Flowrate = 1.5 MGD
Chlorine dose = 2.5 mg/L
Specific weight of 12.5% sodium hypochlorite = 10.2 lbs/gallon
UNKNOWN
Gallons/day of solution
Step 2: CALCULATE LBS/DAY OF PURE CHLORINE REQUIRED
lbs / day = (Flowrate, MGD) x (Dose, mg/L) x (8.34 lbs/gallon)
♦First, enter the flowrate in MGD, and chlorine dose in mg/L.
lbs / day = (1.5 MGD) x (2.5 mg/L) x (8.34 lbs/gallon)
= 31.28 lbs/day of pure chlorine
♦This is the lbs/day of pure chlorine required.
♦However, we’re not using pure chlorine to disinfect the water. Instead, we’re using 12.5% sodium hypochlorite solution, which is weaker than pure chlorine. In fact, the strength of 12.5% sodium hypochlorite solution is only 12.5% of pure chlorine.
♦ Therefore, in STEP 3, we have to convert lbs/day of pure chlorine from STEP 2 to lbs/day of 12.5% sodium hypochlorite.
Step 3: CALCULATE LBS/DAY OF 12.5% SODIUM HYPOCHLORITE REQUIRED
♦Since the strength of 12.5% sodium hypochlorite solution is only 12.5% of pure chlorine, we have to divide the lbs/day of pure chlorine from STEP 2 by .125, which is the decimal format of 12.5%.
♦Therefore, use “31.28 lbs/day of pure chlorine” from STEP 2, and divide it by .125. Consequently, this will give us the lbs/day of 12.5% sodium hypochlorite required.
♦Next, we have to convert 250.2 lbs/day to gallons/day, since the problem statement wants the answer in gallons/day.
Step 4: CALCULATE GALLONS/DAY OF 12.5% SODIUM HYPOCHLORITE REQUIRED
♦Use “250.2 lbs/day of 12.5% sodium hypochlorite” from STEP 3, and divide it by the specific weight of 12.5% sodium hypochlorite, which is 10.2 lbs/gallon. The specific weight tells you how many pounds each gallon of the chemical weighs. To point out, this number was given to us in the problem statement. Using the specific weight, we can convert lbs/day to gallons/day, as shown below.
Therefore, the answer is 24.5 gallons/day.
CONCLUSION
If this was your first time solving this type of water math question, it was probably a bit challenging. However, if you practice solving a lot of these water math questions, you’ll be able to master it.
Moreover, one of the best ways to improve your overall math skills is to solve many water math problems. Hence, repetition is the key to success.
Lastly, it’s also important to solve relevant water math questions. They have to be similar to the ones tested on the operator certification test.
HELPFUL RESOURCES
Practice Tests with Water Math Questions
Online Water Math Course
Finally, if you’re looking for more practice problems, click here.




Pingback: Water Treatment Math Practice Problems - Chemical Dosing Calculations
Pingback: Water Distribution Math Questions - Water and Wastewater Courses